worldwide prevalence ranging between 10% and 30%. Varicose veins can result in a number of symptoms, including pain, leg swelling, itching, and skin discoloration. Individuals with the condition may experience diminished quality of life and loss of work productivity. Finding treatment options that are effective and comfortable for the patient have been an area of interest in the endovascular community. 
![]() Leg of chronic venous insufficiency (CVI), also known as varicose veins, is a relatively common disease, in
Leg of chronic venous insufficiency (CVI), also known as varicose veins, is a relatively common disease, in![]() Worldwide prevalence
Worldwide prevalence![]() Rate between 10% and 30%.Varicose veins can lead to many
Rate between 10% and 30%.Varicose veins can lead to many![]() symptoms
symptoms![]() , including pain, leg swelling, itching and skin color.People can have this kind of situation
, including pain, leg swelling, itching and skin color.People can have this kind of situation![]() experience
experience![]() Lower quality of life and productivity decline.Looking for effective and comfortable treatment scheme has been vascular areas of interest within the industry.
Lower quality of life and productivity decline.Looking for effective and comfortable treatment scheme has been vascular areas of interest within the industry.
A study by researchers in Verona, Italy, found that endovenous mechanochemical ablation (MOCA) treatment with the ClariVein® OC Infusion Catheter was associated with a good occlusion rate, comparable with other techniques, including thermal techniques, without major complications. This is one of the first reports in literature for MOCA treatment of symptomatic varicose veins with 5-year results. With little long-term data pertaining to this type of treatment, the aim of the study was to evaluate long-term occlusion rates." data-gt-human-content="true">A study of researchers in Verona, ItalyFound that usingClariVein ® OC infusion catheterIntravenous mechanical and chemical ablation (MOCA) therapy is associated with good occlusion rate, and other technology (including thermal technology), no major complications.This is the earliest literature about MOCA symptomatic treatment of varicose veins 5 years curative effect is one of the report.Due to the long-term data is rarely associated with such treatment, the aim of this study was to evaluate the long-term rate of occlusion.
Research published in the retrospective single centerVascular surgery magazine: venous and lymphatic disease.Mirandola and others in the outpatient treatment for 395 primary, symptomatic, unilateral, insufficiency of saphenous varicose veins.In these patients, there is no double side treated in the same course.Treatment of vascular most of great saphenous vein (92.3%), and the rest for the small saphenous vein.Surgery using ClariVein OC and 2% of polyethylene glycol single twelve ether liquid preparation.

![]() A total of 329 patients were followed up, each patient 2017 month to 20 years in 2012 received treatment during 18 months.The average follow-up time was 6 + 60 months (range 99.5 1 months).The results show that the technical success rate was 1%.6 cases of incomplete in patients treated with intravenous spasm, catheter were damaged, unable to inject hardener.Including 1 week, XNUMX months follow-up, XNUMX months, XNUMX years of clinical evaluation and duplex ultrasound scanning, and then conducted once a year.
A total of 329 patients were followed up, each patient 2017 month to 20 years in 2012 received treatment during 18 months.The average follow-up time was 6 + 60 months (range 99.5 1 months).The results show that the technical success rate was 1%.6 cases of incomplete in patients treated with intravenous spasm, catheter were damaged, unable to inject hardener.Including 1 week, XNUMX months follow-up, XNUMX months, XNUMX years of clinical evaluation and duplex ultrasound scanning, and then conducted once a year.
Overall survival rate was 92.4% without recanalizationThe anatomical success rate was 94%, 1 year 2 years 3 years was 88%, 91%, 4 years is 88%, 84% for five years.A total of 23 cases were followed up for 5 years, with recanalization (5).The authors note that, in this a series of cases, 36% of cases in the double ultra scanning vein disappear completely.
![]() It is interesting to note that after the 89 patients, the researchers changed the way injection curing agent.Delay with the traditional ClariVein OC technology infusion hardener (scheme 2) than in mechanical pullback PM infusion hardener (scheme 1) will produce a higher rate of occlusion.Scheme 2 results in the accumulation of recanalization survival rate from 92% to 97% (0.013 / P XNUMX XNUMX).
It is interesting to note that after the 89 patients, the researchers changed the way injection curing agent.Delay with the traditional ClariVein OC technology infusion hardener (scheme 2) than in mechanical pullback PM infusion hardener (scheme 1) will produce a higher rate of occlusion.Scheme 2 results in the accumulation of recanalization survival rate from 92% to 97% (0.013 / P XNUMX XNUMX).
Fig 4. Difference in anatomic success between the two different protocols.">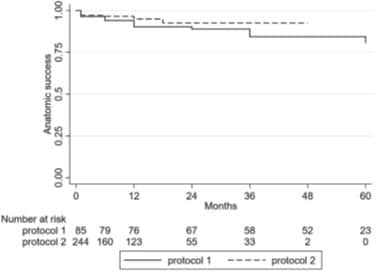
![]() Figure 4. Between the two different schemes of anatomical differences of success.
Figure 4. Between the two different schemes of anatomical differences of success.
">![]() The researchers concluded that ClariVein OC is associated with good occlusion rate, and with other technologies (including thermal technology), no major complications.
The researchers concluded that ClariVein OC is associated with good occlusion rate, and with other technologies (including thermal technology), no major complications.
Varicose veins occur when vessels are unable to pump blood back to the heart, causing the blood to pool, resulting in vessel enlargement over time. Under normal conditions, blood moves from the heart to the legs through the arteries and back to the heart through the veins. To accomplish this, the veins rely on surrounding muscles and a collection of valves to prevent blood from flowing backward. When these muscles and valves weaken or fail to work, blood begins to gather in the vein instead of returning to the heart." data-gt-human-content="true">When blood vessels can not to pump the blood back to the heart, blood, increases over time lead to blood vessels, varicose veins.Under normal circumstances, the blood through the arteries flowing from the heart to the leg, and then through the venous return to the heart.In order to achieve this goal, rely on the surrounding muscles and a series of venous valves to prevent blood flow backwards.When these muscles and valve weaken or unable to work, began to gather in the venous blood, rather than return to the heart.
other symptoms of varicose veins include:" data-gt-human-content="true">Varicose veins in addition to pain, swelling, itching and skin color,Other symptomsInclude:
 Distortion and ballooning vein
Distortion and ballooning vein Pain, heat, stirring, and muscle spasm
Pain, heat, stirring, and muscle spasm The leg heavy and fatigue
The leg heavy and fatigue
![]() ClariVein OC by professional function of infusion catheter combined with rotating godet tips, to control the d specified in the target dispersion within the blood vessels of 360 degrees, easing the symptoms.Use ClariVein OC treatment need only through the skin an entrance to the needle size, need a very short time, do not use hot or swelling anesthesia, cause the smallest discomfort to the patients.
ClariVein OC by professional function of infusion catheter combined with rotating godet tips, to control the d specified in the target dispersion within the blood vessels of 360 degrees, easing the symptoms.Use ClariVein OC treatment need only through the skin an entrance to the needle size, need a very short time, do not use hot or swelling anesthesia, cause the smallest discomfort to the patients.
ClariVein.com or contact our bob sports for more information." data-gt-human-content="true">Details about after clinical validation of ClariVein OC and how it changed the lives of patients.accessClariVein.comOr contact usCustomer supportFor more information.
![]() Refer to the article:
Refer to the article:
![]() Mirandola M et al. 2020. "An Italian Experience with Mechanochemical Ablation of the Saphenous Vein Since 2012." J Vasc Surg Venous Lymphat Disord 8, no. 6 (Nov) : 999-1005. PMID: 32179039.
Mirandola M et al. 2020. "An Italian Experience with Mechanochemical Ablation of the Saphenous Vein Since 2012." J Vasc Surg Venous Lymphat Disord 8, no. 6 (Nov) : 999-1005. PMID: 32179039.